Coating Viability
Surface energy [: defined as the excess energy at the
surface of a material compared to the bulk or the work per unit area done
by the force that creates the new surface]
Interface energy) [: in the physics of solids, surfaces must
be intrinsically less energetically favourable than the bulk of a material (the
molecules on the surface have more energy compared with the molecules in the
bulk of the material]
The hydrophobic properties of a surface
can be measured by its contact angle [:
the higher the contact angle the higher the hydrophobicity of a surface.
Surfaces with a contact angle < 90° are referred to as hydrophilic and those
with an angle >90° as hydrophobic]
The intrinsic hydrophobicity of a
surface can be enhanced by being textured with different length scales of
roughness. The red rose takes advantage of this by using a hierarchy of micro-
and nanostructures on each petal to provide sufficient roughness for super
hydrophobicity. More specifically, each rose petal has a collection of
micropapillae on the surface and each papilla, in turn, has many nanofolds.
The
term “petal effect” describes the fact that a water droplet on the surface of a
rose petal is spherical in shape, but cannot roll off even if the petal is
turned upside down. The water drops maintain their spherical shape due to the
super hydrophobicity of the petal (contact angle of about 155°), but do not
roll off because the petal surface has a high adhesive force with water.
When comparing the "petal
effect" to the "lotus effect", it is important to note some
striking differences. The surface structure of the lotus petal and the rose
petal produce two different effects. The lotus petal has a randomly rough
surface and low contact angle hysteresis, which means the water droplet is not
able to wet the microstructure spaces between the spikes. This allows air to
remain inside the texture, causing a heterogeneous surface composed of both air
and solid. As a result, the adhesive force between the water and the solid
surface is extremely low, allowing the water to roll off easily (i.e.
"self-cleaning" phenomenon).
However, the rose petals micro- and
nanostructures are larger in scale than those of the lotus leaf, which allows
the liquid film to impregnate the texture. However, the liquid can enter the
larger-scale grooves, but it cannot enter into the smaller grooves. This is
known as the Cassie impregnating wetting regime. Since the liquid can wet the
larger-scale grooves, the adhesive force between the water and solid is very
high. This explains why the water droplet will not fall off even if the petal
is tilted at an angle or turned upside down. However, this effect will fail if
the droplet has a volume larger than 10 µl because the balance between weight
and surface tension is surpassed.
High or Low energy surfaces
Liquids can interact with two main types
of solid surfaces. Traditionally, solid surfaces have been divided into
high-energy solids and low-energy types. The relative energy of a solid has to
do with the bulk nature of the solid itself. Solids such as metals, glasses,
and ceramics are known as 'hard solids' because the chemical bonds that hold
them together (e.g., covalent, ionic, or metallic) are very strong. Thus, it
takes a large input of energy to break these solids, so they are termed high energy
The other type of solids is weak
molecular crystals (polyurethane’s) where the molecules are held together
essentially by physical forces (e.g., van der Waals and hydrogen bonds). Since
these solids are held together by weak forces, a very low input of energy is
required to break them, thus they are termed low
energy
Surface energy and wetting
For optimum adhesion, a coating must
thoroughly wetting the surface to be bonded. Wetting means the coating flows
and covers a surface to maximize the contact area and the attractive forces
between the coating and bonding (paint) surface.
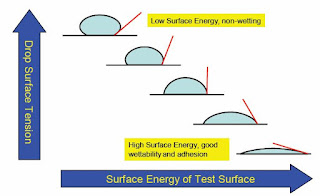
A lower surface energy material, such as
water, will spontaneously wet out a higher energy surface, such as the un-waxed
hood of a car. A waxed hood, however, has a lower surface energy than water.
The water beads up rather than wet out, reducing its contact area with the
surface.
In order to successfully form a
continuous coating, the liquid should be able to wet the surface of the
material. Wettability depends on one specific property of the surface: surface
energy. The surface energy of the solid substrate directly affects how well a
liquid wets the surface. To achieve good wettability the surface energy of the
substrate needs to exceed that of the surface tension of the liquid by around 2
- 10 mN/m (Dynes). The Dyne level reflects the surface wettability - the higher
the Dyne level, the better the wettability/adhesion.
To obtain optimum adhesion, it is
necessary to increase the surface energy of the substrate to just above that of
the material to be applied. For optimum adhesion of a coating on various
substrates, it is necessary to obtain a high surface energy. Determining the
surface energy can be achieved by measuring contact angle or by use of Surface
Energy Test Liquids or Pens (Dyne level testing).
This form of measurement is based on the ISO method for measuring the surface energy of polyethylene film. Surface energy may be defined as the excess energy at the surface of a material compared to the bulk. Every solid surface has a specific and measurable surface energy. The unit of measurement used is the Dyne/cm² or mN/m.
When the Dyne level test liquid is
applied to the surface, the liquid will either form a continuous film on the
surface or pull back into small droplets. If the Dyne test fluid remains as a
film for 3 seconds, the substrate will have a minimum surface energy of that
fluid value, expressed in N/m (Dynes). Should the Dyne test fluid reticulate or
draw back into droplets in less than 1 second then the surface energy of the
substrate is lower than that of the fluid itself. To test the viability of a
coating requires measuring the surface energy (surface tension).
Using a Dyne Test Pen will measure the surface tension and identify whither the
coating is still viable, which requires a surface tension of 38mN/m or higher.
Measure the surface energy by using
slight pressure to draw the Dyne Quick Test Pen tip across the coated surface.
If the ink lines shrink or bead within 1-2 seconds then the surface protection
is degrading as the surface energy level has dropped to less than 38 Dynes. If
the ink lines remain as marked and do not shrink then the test sample surface
protection is still viable as the coating has a surface energy of 38 Dynes or
higher
.
Dyne Quick Test Pen - http://www.dynetechnology.co.uk/measurement-equipment/quick-test-pens/
Always be willing to learn; because the
more you learn, the more you’ll realize what you don’t know.
It is said that knowledge is power, with
the caveat that it includes access to a reliable information sources. I would
like to think that these articles become an asset to anyone who is new to
detailing and to professional’s alike, as well as industry experts who seek to
advance their knowledge.
I hope the article are informative. By
having some understanding of the ‘What’ and ‘Why’ as well as the ‘How’ along
with a little science to help you understand how the chemicals we use react,
you can achieve the results you desire.
I would appreciate it if you would share
these articles as it helps other detailers further their knowledge.
This was a tough subject to explain, so
I hope this makes sense and helps you. As always if you have questions, I’ll do
my best to answer; bear in mind the only stupid questions is the one that was
unasked. Questions and/ or constructive comments are always appreciated.
Copyright
© 2002 - 2015 TOGWT® (Established 1980) all rights reserved





No comments:
Post a Comment